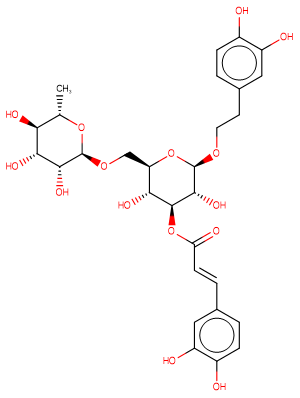
Forsythoside I
CAS No. 1177581-50-8
Forsythoside I( —— )
Catalog No. M21477 CAS No. 1177581-50-8
Forsythoside I a caffeoyl phenylethanoid glycoside (CPG) may possess anti-inflammatory activities.
Purity : >98% (HPLC)
 COA
COA
 Datasheet
Datasheet
 HNMR
HNMR
 HPLC
HPLC
 MSDS
MSDS
 Handing Instructions
Handing Instructions
| Size | Price / USD | Stock | Quantity |
| 5MG | 99 | In Stock |


|
| 100MG | Get Quote | In Stock |


|
| 200MG | Get Quote | In Stock |


|
| 500MG | Get Quote | In Stock |


|
| 1G | Get Quote | In Stock |


|
Biological Information
-
Product NameForsythoside I
-
NoteResearch use only, not for human use.
-
Brief DescriptionForsythoside I a caffeoyl phenylethanoid glycoside (CPG) may possess anti-inflammatory activities.
-
DescriptionForsythoside I a caffeoyl phenylethanoid glycoside (CPG) may possess anti-inflammatory activities.
-
In Vitro——
-
In Vivo——
-
Synonyms——
-
PathwayOthers
-
TargetOther Targets
-
RecptorOthers
-
Research Area——
-
Indication——
Chemical Information
-
CAS Number1177581-50-8
-
Formula Weight624.6
-
Molecular FormulaC29H36O15
-
Purity>98% (HPLC)
-
SolubilityIn Vitro:?DMSO : 100 mg/mL (160.11 mM)
-
SMILESC[C@@H]1O[C@@H](OC[C@H]2O[C@@H](OCCc3ccc(O)c(O)c3)[C@H](O)[C@@H](OC(=O)\C=C\c3ccc(O)c(O)c3)[C@@H]2O)[C@H](O)[C@H](O)[C@H]1O
-
Chemical Name——
Shipping & Storage Information
-
Storage(-20℃)
-
ShippingWith Ice Pack
-
Stability≥ 2 years
Reference
1.Wang FN et al. New phenylethanoid glycosides from the fruits of forsythia suspense (thunb.) vahl. Molecules. 2009 Mar 25;14(3):1324-31.
molnova catalog



related products
-
PK150
PK150 shows oral bioavailability and antibacterial activity against several pathogenic strains at submicromolar concentrations.
-
Fibrinogen Binding I...
Fibrinogen Binding Inhibitor Peptide, a synthetic dodecapeptide, represents the specific platelet receptor recognition site of the human fibrinogen g-chain (residues 400-411).
-
Potassium thioacetat...
Potassium thioacetate is widely used as a sulfur source in the synthesis of sulfur-containing organic compounds.



 Cart
Cart
 sales@molnova.com
sales@molnova.com


